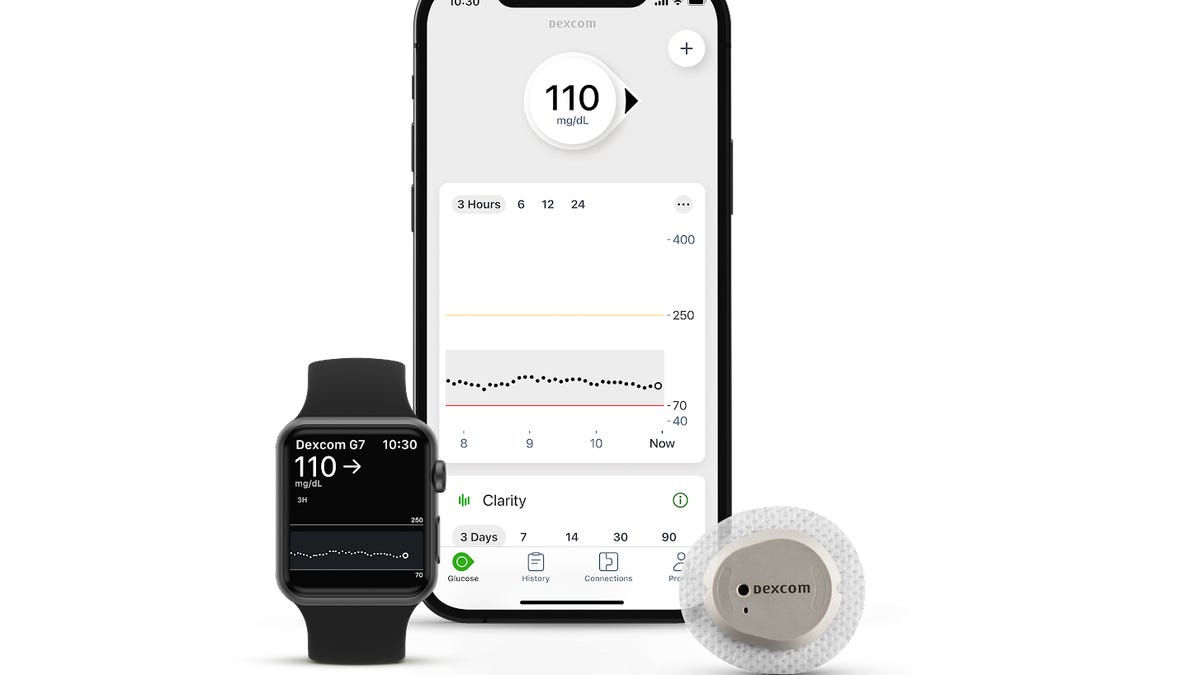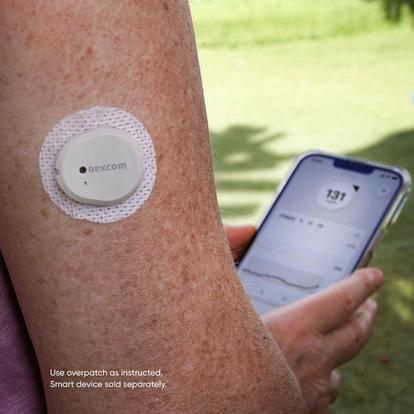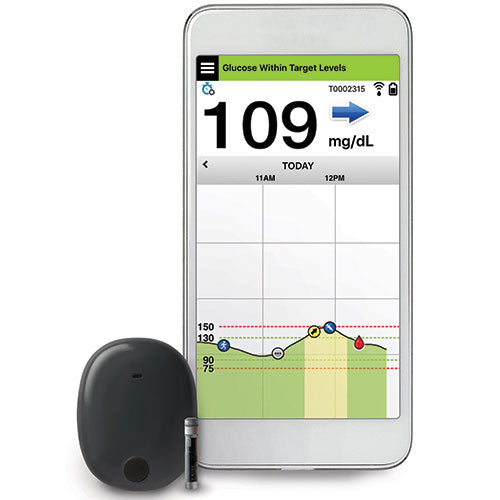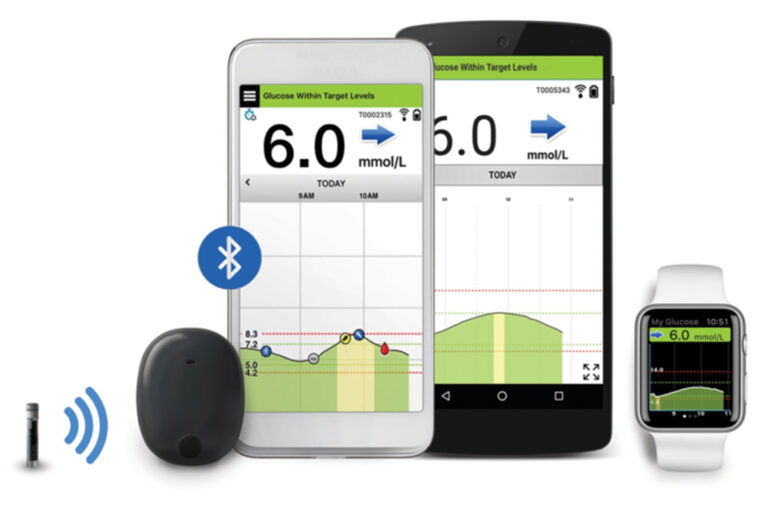
Eversense E3 CGM Approved for Two Sensors per Year: Your “Happily Ever(sense) After” - Taking Control Of Your Diabetes®

OneTouch Vibe Plus Insulin Pump Earns FDA Approval And Health Canada License And Is First Pump Integrated With The Dexcom G5 Mobile Continuous Glucose Monitor

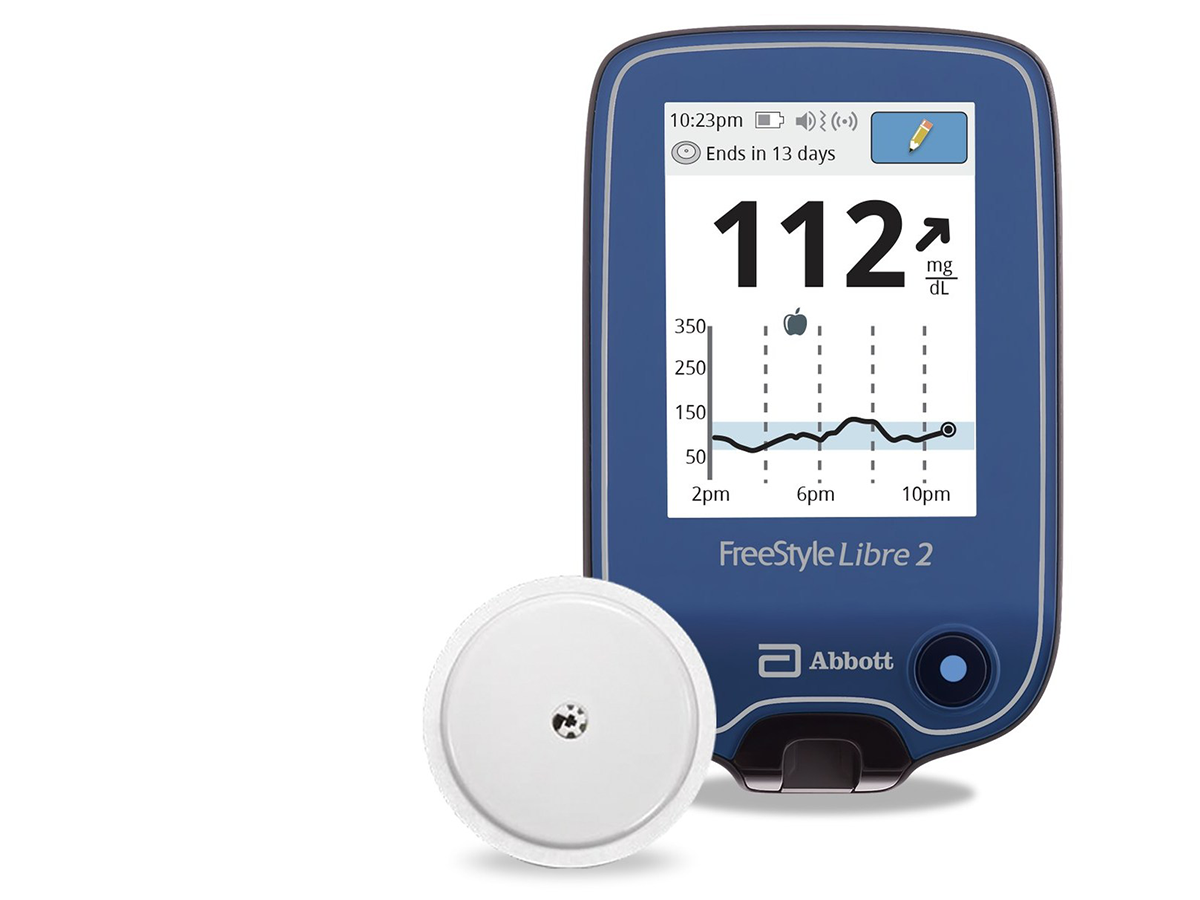
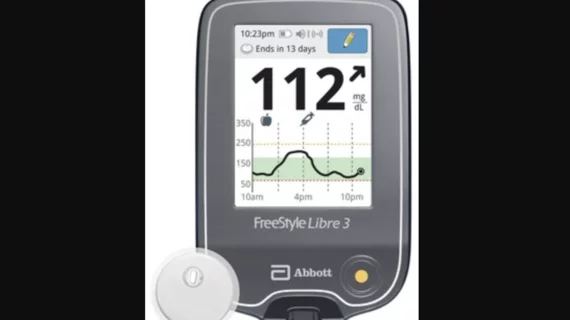
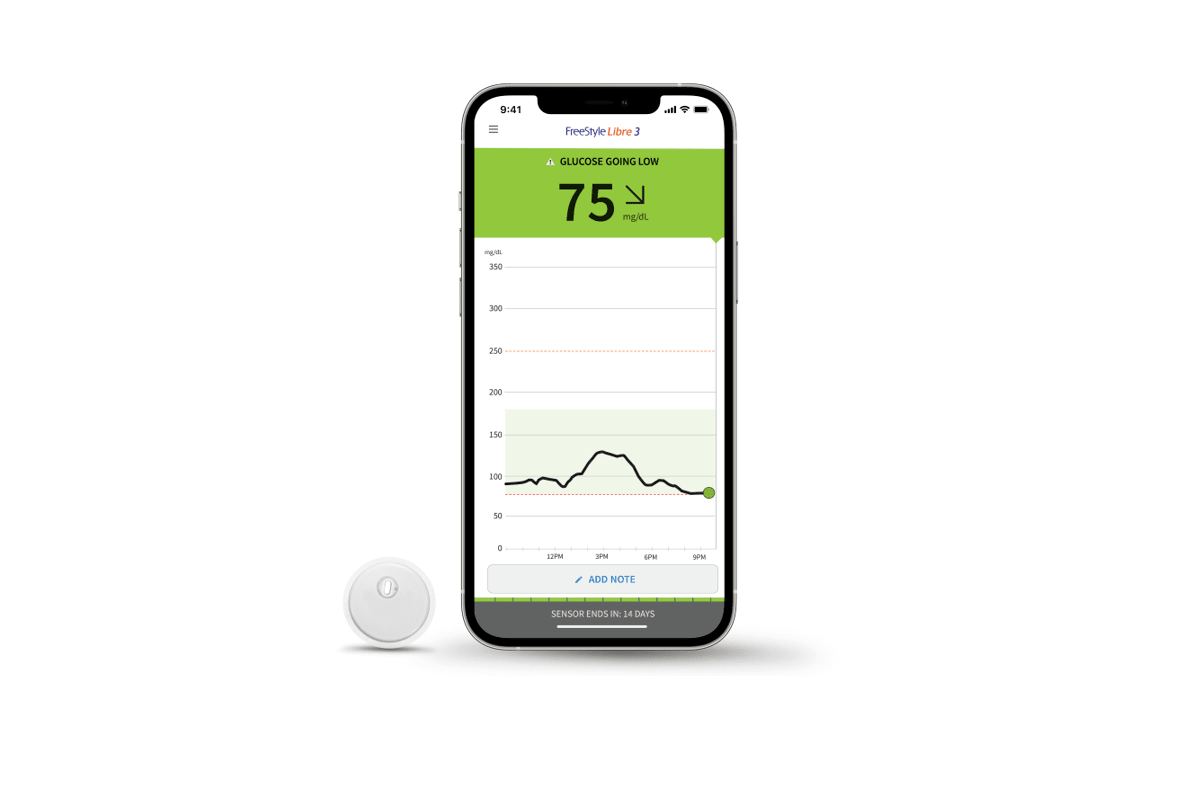
Abbott's Freestyle Libre system becomes first CGM to be FDA cleared for use without fingersticks | MobiHealthNews
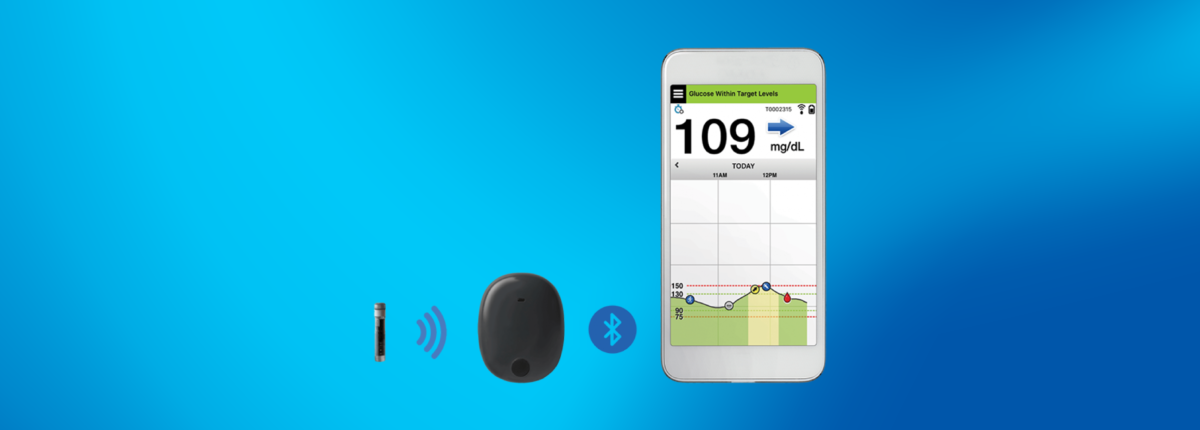
FDA Approves First Continuous Glucose Monitoring System for Adults Not Requiring Blood Sample Calibration - Medical Design and Outsourcing

The World's Smallest CGM Gets FDA Approval, and Bigger Isn't Always Better! - Taking Control Of Your Diabetes®

FDA Panel 8-2 Vote Backs Continuous Glucose Monitoring to Replace Fingersticks - Legacy MedSearch | Medical Device Recruiters

FDA approves Eversense E3 6-month continuous glucose monitor that requires fewer fingerstick blood glucose measurements - NotebookCheck.net News

FDA approves first continuous glucose monitoring system to replace fingerstick test in diabetes - Clinical Advisor