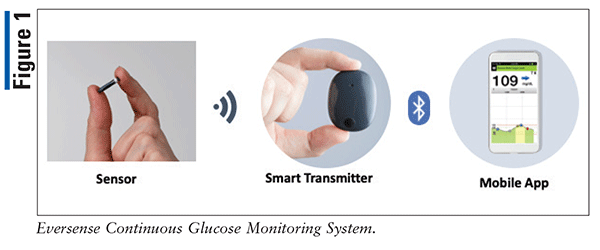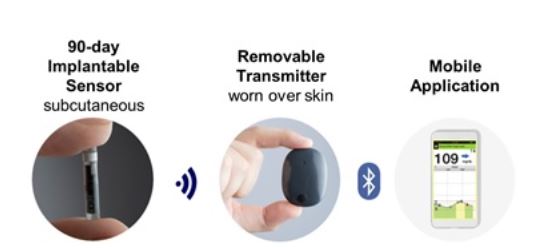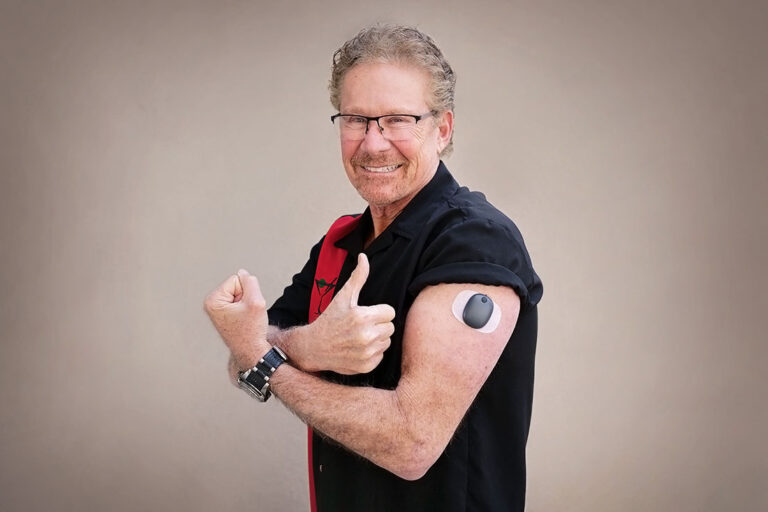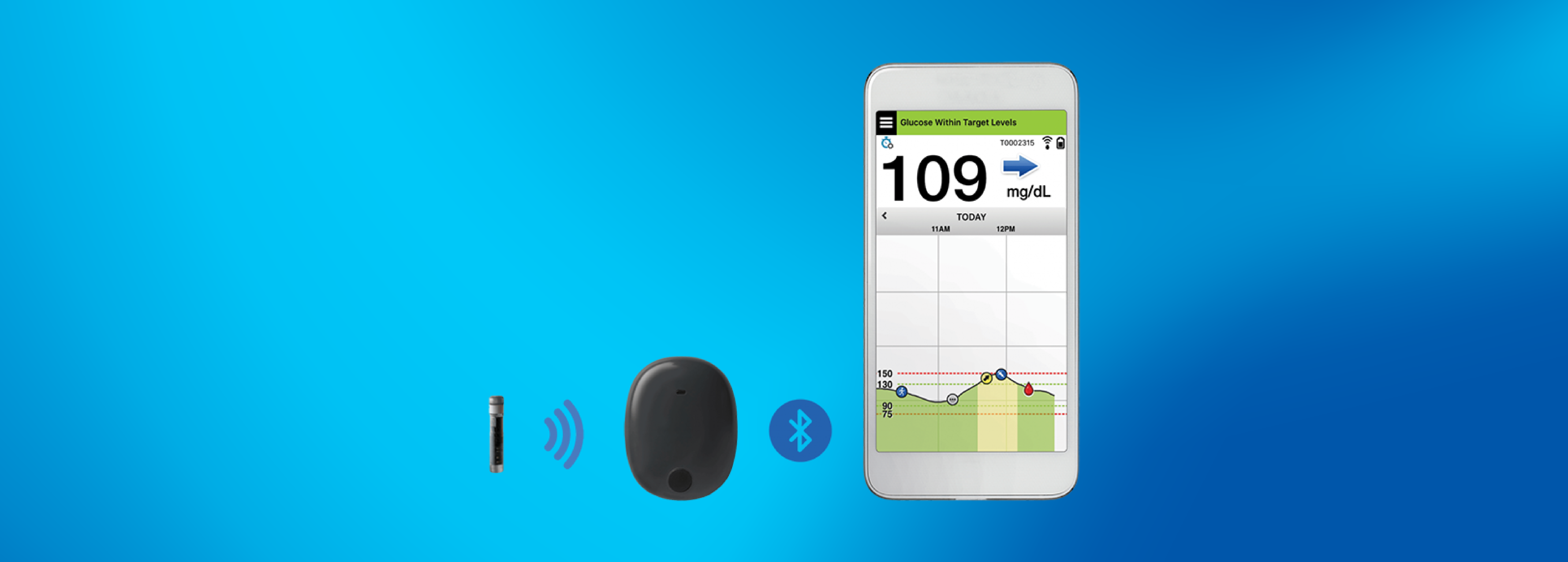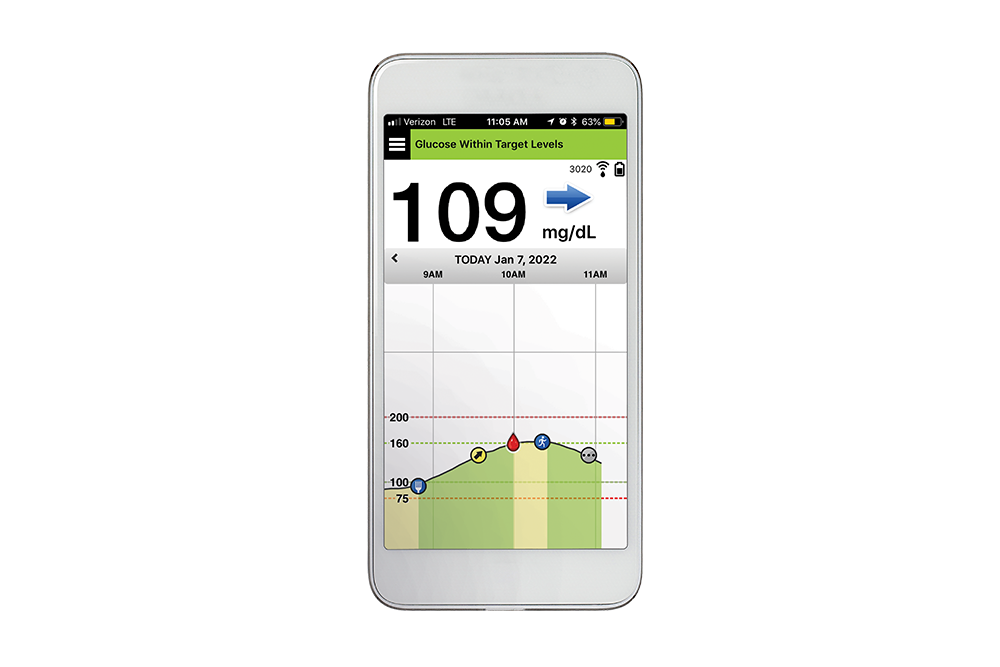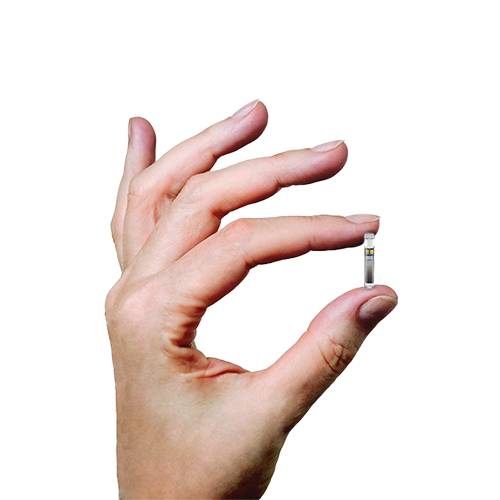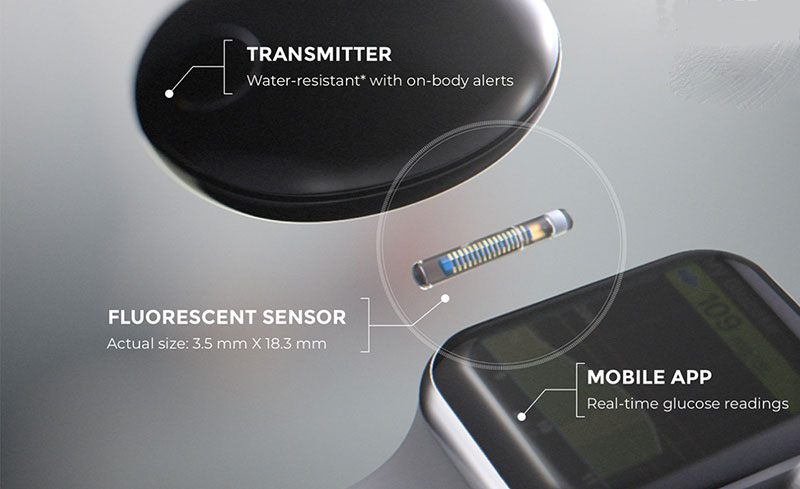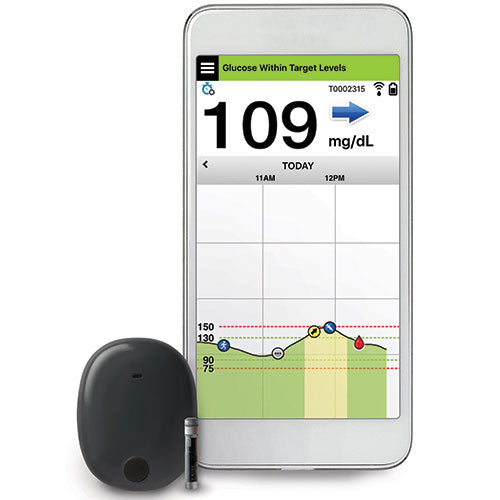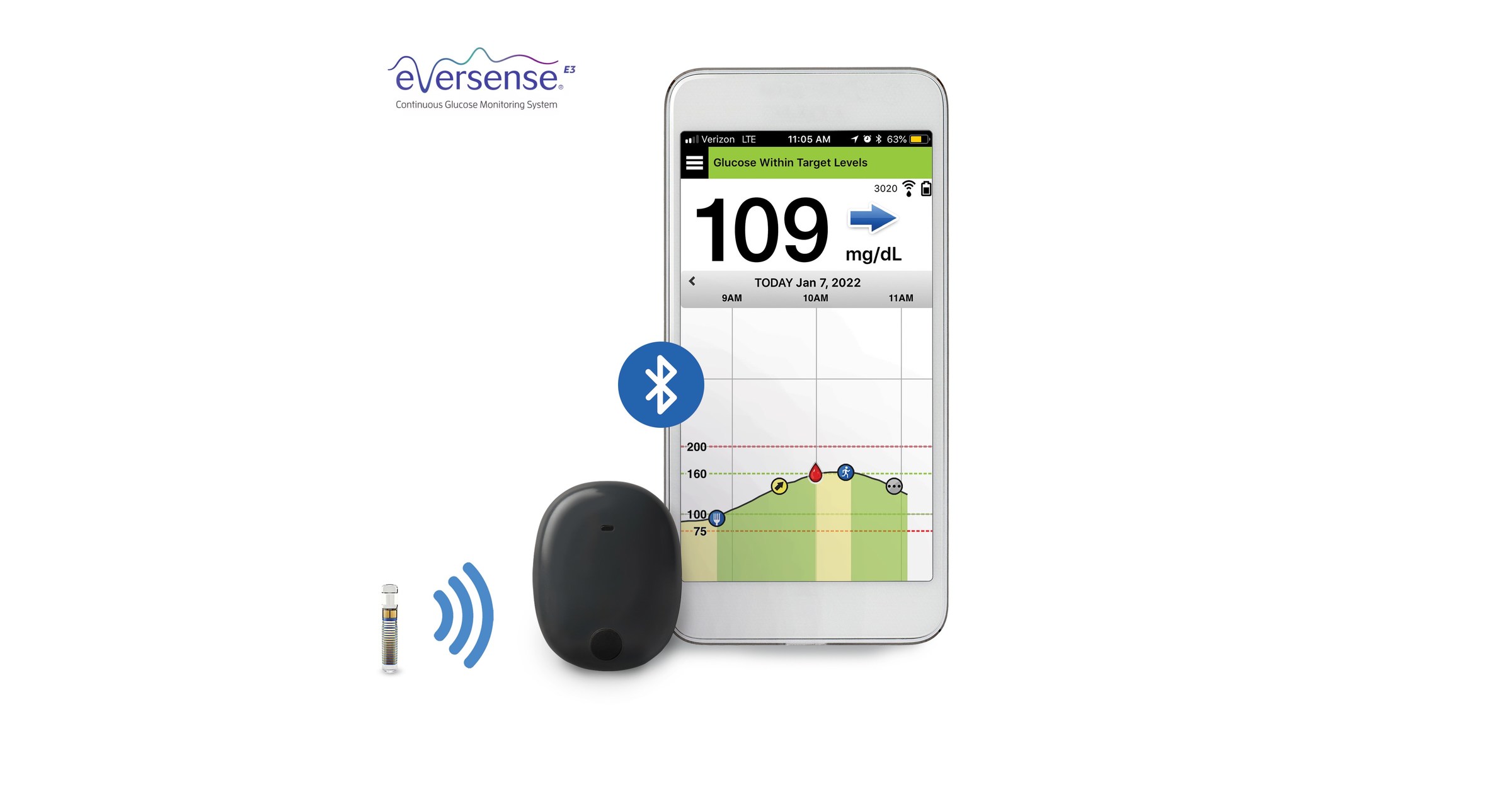
ASCENSIA DIABETES CARE LAUNCHES THE 6 MONTH EVERSENSE® E3 CONTINUOUS GLUCOSE MONITORING SYSTEM IN THE U.S. WITH THE NEW EVERSENSE PASS SAVINGS PROGRAM
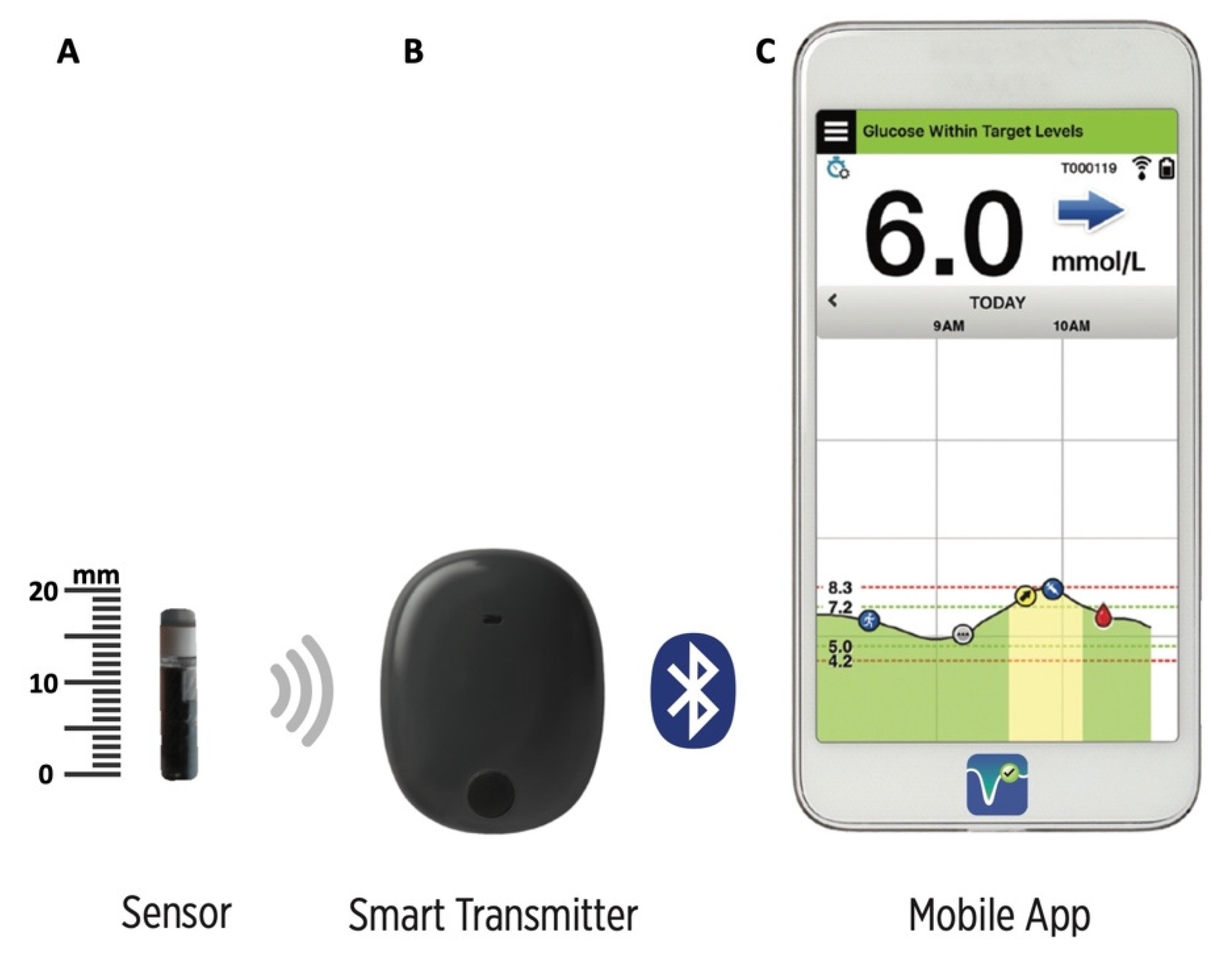
Animals | Free Full-Text | Clinical Use of a 180-Day Implantable Glucose Monitoring System in Dogs with Diabetes Mellitus: A Case Series
Senseonics And Beta Bionics Partner To Develop The Bionic Pancreas System | Medical Product Outsourcing